
Coronavirus
What is the latest news on the coronavirus vaccines?
5 years ago
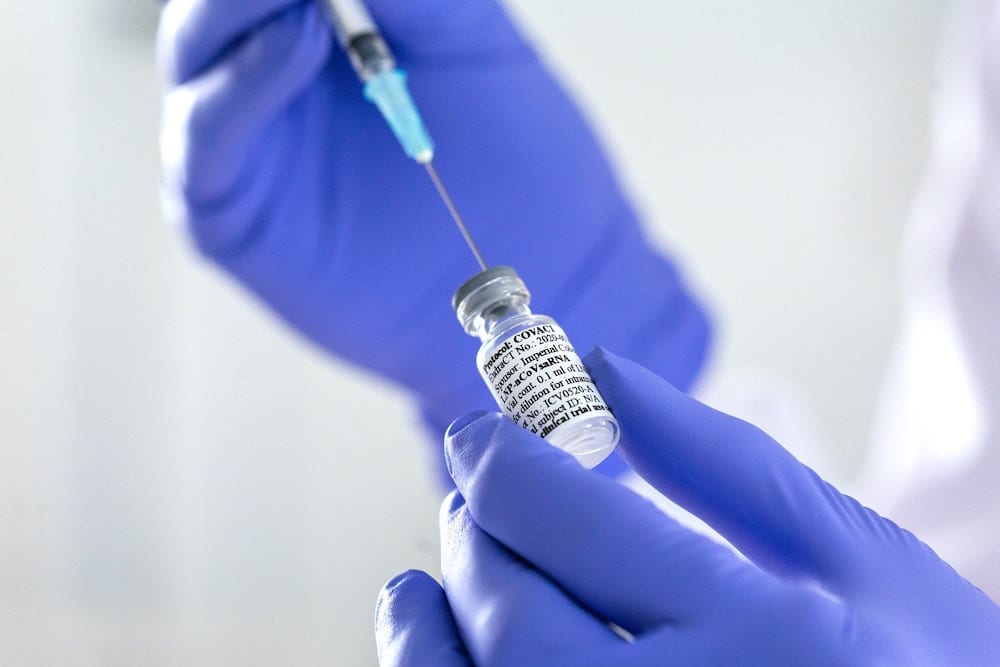
After successful trials, coronavirus vaccines could be rolled out at GP surgeries, pharmacies and mass testing centres.
Covid-19 vaccines could be rolled out in the UK within the first half of next year, with the NHS to prepare itself to deliver doses by Christmas “if they become available”.
After successful trials, vaccines could be rolled out at GP surgeries, pharmacies and mass testing centres.
But health chiefs say a mass vaccination programme is unlikely to get under way before next year.
There are currently more than 200 coronavirus vaccine candidates being tested around the world.
Here is everything you need to know about the race to get a Covid-19 vaccine.
What progress is being made with Covid-19 vaccines?
A total of 44 of the vaccine candidates in development are at clinical trial stage.
Of these, nine are in the phase three stage of clinical evaluation and are being given to thousands of people to confirm safety and effectiveness.
There are two frontrunners in the Covid-19 vaccine race – one from German biotech firm BioNtech and US pharmaceutical company Pfizer, and another being developed by the University of Oxford and AstraZeneca.
Both vaccines are currently in phase three clinical trials.
The Oxford vaccine, called ChAdOx1 nCoV-19, uses a weakened version of a common cold virus (adenovirus) which causes infections in chimpanzees.
Other potential vaccines in phase three trials include ones by US drugs firm Moderna and biotech company Novavax.
What trials are ongoing in the UK?
Aside from the Oxford vaccine, a coronavirus jab is being developed by Imperial College London.
The Imperial vaccine is in phase one of clinical testing, where doses are being given to a small group of people to determine whether it is safe and to learn more about the immune response it provokes.
Pharmaceutical companies Sanofi and GlaxoSmithKline have also teamed up with the hope of making a Covid-19 vaccine available by the middle of next year.
The Sanofi/GSK candidate is in the phase two stage, where the vaccine is being given to hundreds of people so scientists can learn more about its safety and correct dosage.
They plan to begin phase three trial by the end of the year.

When will the results from these trials be available?
The head of the UK’s vaccines taskforce, Kate Bingham, said data from the vaccine trials at the University of Oxford and AstraZeneca, and Pfizer with BioNTech, could be available this year.
She said if she puts on “rose-tinted specs” she would hope to see positive interim data from both Oxford and Pfizer BioNtech in early December.
Professor Andrew Pollard, head of Oxford’s vaccine trial team, said he is optimistic data on safety and efficacy of their vaccine will be available by the end of the year.
Professor Robin Shattock, who is leading Imperial College London’s Covid-19 vaccine effort, said data on its efficacy will be available in the middle of next year.

Does the UK have access to any of these potential vaccines?
In August, the Government announced the UK has secured access to six Covid-19 vaccine candidates in development, representing 340 million doses.
Ms Bingham said there should be about four million vaccine doses available by the end of the year.
The UK has also secured 30 million doses of the vaccine being developed by BioNtech and Pfizer.
The deals cover four different types of vaccines – adenoviral vaccines, mRNA vaccines, inactivated whole virus vaccines and protein adjuvant vaccines.
Adenoviral vaccines are weakened versions of adenoviruses, while mRNA candidates are made up of small or inactivated doses of the whole disease-causing organism.
Inactivated whole virus vaccines, on the other hand, contain whole bacteria or viruses which have been killed, while protein adjuvant jabs are those where an adjuvant is added to enhance the immune response.
Should any of these candidates be approved, the most vulnerable, the elderly, people living in care homes, and health and social care staff will be front of the queue to receive a jab, followed by those who are high at risk.
When will a coronavirus vaccine become available?
A vaccine usually takes years, often decades, to develop but scientists working on potential coronavirus jabs are hoping to achieve the same amount of work in a few months.
Most experts are optimistic that a vaccine is likely to become available by mid-2021, which would be around 12-18 months after the new coronavirus first emerged.
NHS England chief executive Sir Simon Stevens said the “expectation” is that any vaccination programme would begin in the new year – pending positive results from clinical trials.
Meanwhile, Ms Bingham said she has 50% confidence that by Easter or early summer next year, all vulnerable people in the country will have a vaccine.
Prof Pollard said clinical trials would need to take place in the child population before Covid-19 vaccines can be given to youngsters.
He said: “Those trials are being planned, but at the moment we do not have any data about immune response or the safety of children, and so that is something which has to be done through the normal scientific process, and I would anticipate that that will happen towards the end of this year or during the early part of next year.”

Where will the vaccines be administered?
Sir Simon said a potential vaccination programme will see vaccines delivered at GP surgeries, pharmacies and mass testing centres – including at the Nightingale hospitals.
He said GPs will be put on standby from December should a vaccine be made available before Christmas.








 Subscribe
Subscribe Follow Us
Follow Us Follow Us
Follow Us Follow Us
Follow Us Follow Us
Follow Us Follow Us
Follow Us











